Ex vivo experiments are conducted on clinical samples, utilizing innovative laboratory assays and advanced computational approaches to explore disease pathways and biomarkers.
Autoantibody Responses in the COVID-19 Host Immune Response Pathogenesis (CHIRP) Study
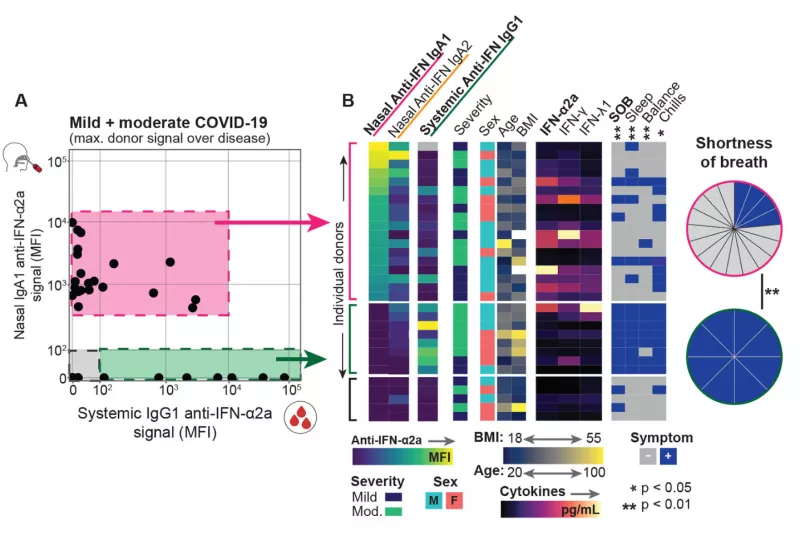
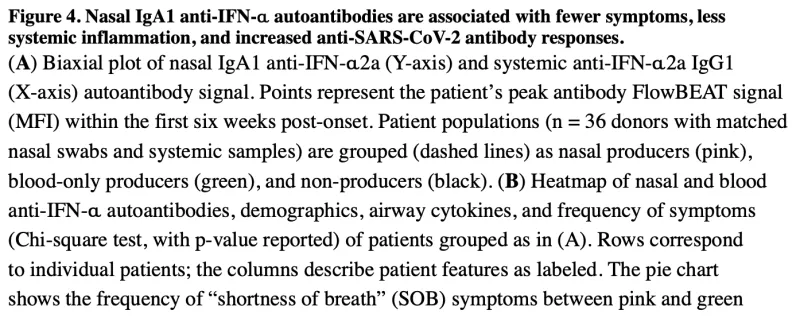
The COVID-19 pandemic caused devastating morbidity and mortality worldwide. However, the virus itself does not explain the person-to-person variability in clinical course (ranging from asymptomatic carriage to severe lethal respiratory disease, as well as long-term inflammatory sequelae). We developed the acute COVID-19 Host Immune Response Pathogenesis (CHIRP) study to obtain convalescent samples from people infected with SARS-CoV-2 virus. We hypothesized that individual variation in host responses likely play a major role in determining the severity of COVID-19 as well as the ability for the host to recover from disease. Performing in-depth sampling of blood, sputum, nasal swab, saliva, urine, and stool we applied a novel assay (FlowBEAT) developed by our collaborators and profiled the isotypes of anti-SARS-CoV-2 and anti-interferon alpha (anti-IFN-α) antibodies from longitudinal samples. We hypothesized that anti-IFN-α autoantibodies in the airways, the initial site of infection, might determine COVID-19 severity. We found that nasal IgA1 anti-IFN-α autoantibodies were induced post-infection onset in more than 70% of mild and moderate COVID-19 cases and were associated with robust anti-SARS-CoV-2 immunity, fewer symptoms, and efficient recovery. Nasal anti-IFN-α autoantibodies followed the peak of host IFN-α production and waned with disease recovery, revealing a regulated balance between IFN-α and anti-IFN-α response. In contrast, systemic IgG1 anti-IFN-α autoantibodies appeared later and were detected only in a subset of patients with elevated systemic inflammation and worsening symptoms. These data reveal a protective role for nasal anti-IFN-α in the immunopathology of COVID-19 and suggest that anti-IFN-α autoantibodies may serve a homeostatic function to regulate host IFN-α following viral infection in the respiratory mucosa.
HIV Reservoir Decay during Acute Treated HIV
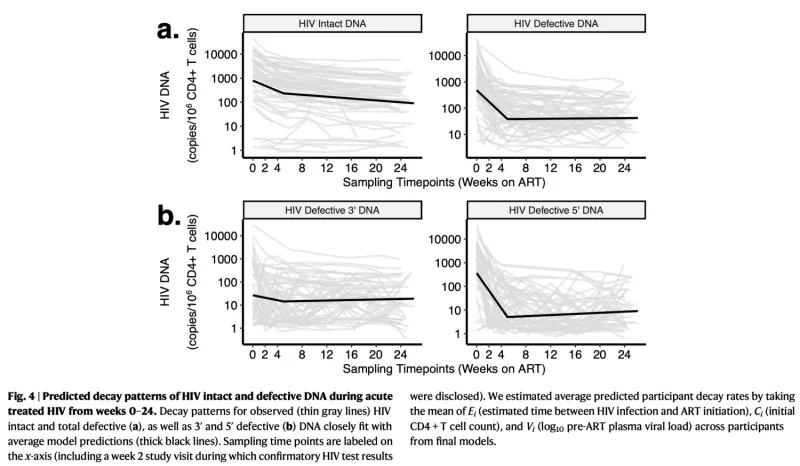
Despite over four decades of research, we do not yet have an HIV cure. While antiretroviral therapy (ART) is able to suppress virus to undetectable levels, virus rapidly rebounds from latently-infected cells (“the HIV reservoir”) within weeks of ART interruption. The goal of HIV cure is to accelerate the decay of the reservoir and elicit a “functional cure” (e.g., recapitulating “elite controllers”, or people treated during or very early after infection, “post-treatment controllers”, who are able to suppress virus in the absence of ART). The majority of HIV cure trials to date have been unsuccessful, but these trials have largely included people treated during chronic HIV (>2 years from infection; thus, immune responses may have been exhausted and dysfunctional). Leveraging >500 longitudinal blood samples, we developed a mathematical model of reservoir decay among 67 people with HIV (PWH) from the UCSF Treat Acute HIV cohort (initiating ART <100 days of HIV infection) and fit various mono-, bi-, and triphasic decay curves. For both HIV intact (infected cells harboring intact viral sequences able to produce infectious virions) and defective (the majority of the HIV reservoir but incapable of producing infectious virions) DNA, we observed biphasic decay patterns for both measures. Furthermore, both HIV intact and defective DNA decay rates were significantly faster among PWH with known clinical factors associated with enhanced host viral control: higher initial CD4+ T cell count, earlier initiation of ART, and lower pre-ART viral loads. As further validation of our mathematically modeling approach, we also fit decay models for plasma HIV RNA (viral load measured at each study visit using a standard clinical assay with limit of detection < 40 copies/mL). We observed a triphasic decay of plasma HIV RNA, similar to prior reports among PLWH initiating ART. This approach may also help inform the design of future HIV cure trials, e.g., to predict optimal timeframes during which an intervention may have the greatest impact on accelerating reservoir decay and/or limiting reservoir establishment.
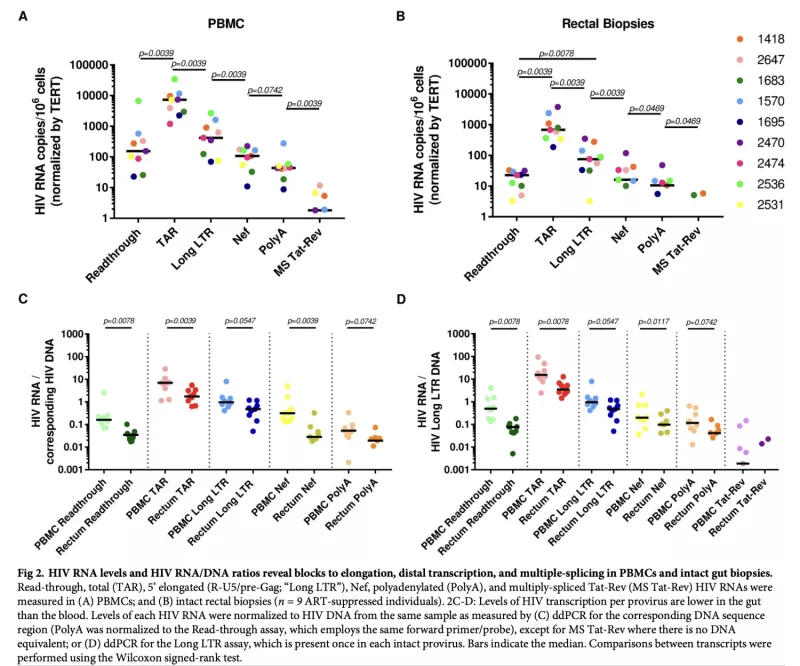
Tissue-Specific Differences in HIV Reservoir Transcription during ART Suppression
Latently-infected CD4+ T cells are widely considered to be the major barrier to a cure for HIV. Much of our understanding of HIV latency comes from latency models and blood cells, but most HIV-infected cells reside in lymphoid tissues such as the gut. We hypothesized that tissue-specific environments may impact the mechanisms that govern HIV expression. Leveraging samples from PWH from our UCSF SCOPE HIV cohort, we assessed the degree to which different mechanisms inhibit HIV transcription in the gut and blood. We quantified HIV transcripts suggestive of transcriptional interference (U3-U5; "Read-through"), initiation (TAR), 5’ elongation (R-U5-pre-Gag; "Long LTR"), distal transcription (Nef), completion (U3-polyA; "PolyA"), and multiple splicing (Tat-Rev) in matched peripheral blood mononuclear cells (PBMCs) and rectal biopsies, and matched FACS-sorted CD4+ T cells from blood and rectum. Like the PBMCs, rectal biopsies showed low levels of read-through transcripts (median = 23 copies/106 cells) and a gradient of total (679) > elongated (75) > Nef (16) > polyadenylated (11) > multiply-spliced HIV RNAs(<1), P<0.05 for all. This demonstrated that there were potential blocks to HIV transcriptional elongation, completion, and splicing. Rectal CD4+ T cells showed a similar gradient of total>polyadenylated>multiply-spliced transcripts, but the ratio of total to elongated transcripts was 6-fold lower than in blood CD4+ T cells (P = 0.016), suggesting less of a block to HIV transcriptional elongation in rectal CD4+ T cells. Levels of total transcripts per provirus were significantly lower in rectal biopsies compared to PBMCs (median 3.5 vs. 15.4; P = 0.008) and in sorted CD4+ T cells from rectum compared to blood (median 2.7 vs. 31.8; P = 0.016). The lower levels of HIV transcriptional initiation and of most HIV transcripts per provirus in the rectum suggest that this site may be enriched for latently-infected cells, cells in which latency is maintained by different mechanisms, or cells in a "deeper" state of latency. These findings highlight important considerations in the future design of therapies that aim to disrupt HIV latency in all tissue compartments.
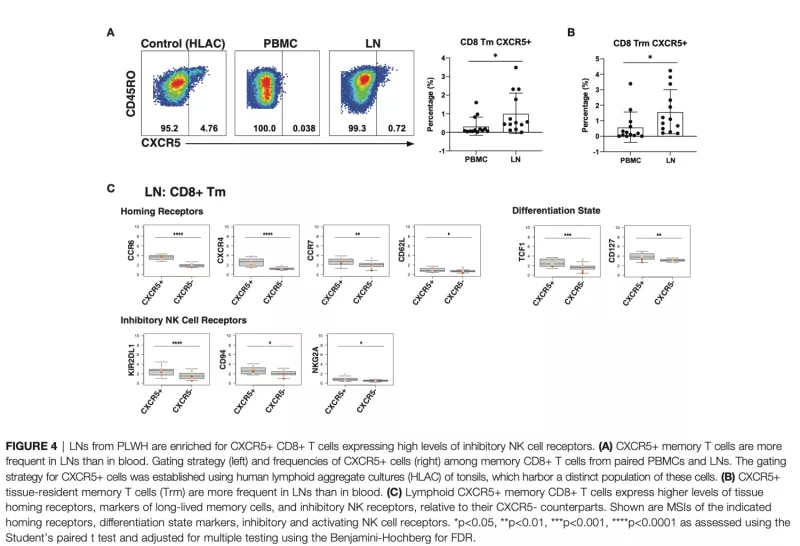
Immune Cell Phenotypes from Lymph Node Aspirates from HIV Controllers and ART-Suppressed Non-Controllers
T and natural killer (NK) cells are effector cells with key roles in anti-HIV immunity, including in lymphoid tissues, the major site of HIV persistence. However, little is known about the features of these effector cells from people with HIV (PWH), particularly from those who initiated antiretroviral therapy (ART) during acute infection. Our study design was to use 42-parameter CyTOF to conduct deep phenotyping of paired blood- and lymph node (LN)-derived T and NK cells from three groups of aviremic PWH: elite controllers (N = 5), and ART-suppressed PWH who had started therapy during chronic (N = 6) vs. acute infection (N = 8), the latter of which is associated with better outcomes. We found that acute-treated individuals are enriched for speci c subsets of T and NK cells, including blood-derived CD56-CD16+ NK cells previously associated with HIV control, and LN-derived CD4+ T follicular helper cells with heightened expansion potential. An in-depth comparison of the features of the cells from blood vs. LNs of PWH from our cohort revealed that T cells from blood were more activated than those from LNs. By contrast, LNs were enriched for follicle-homing CXCR5+ CD8+ T cells, which expressed increased levels of inhibitory receptors and markers of survival and proliferation as compared to their CXCR5- counterparts. In addition, a subset of memory-like CD56brightTCF1+ NK cells was enriched in LNs relative to blood. These results together suggest unique T and NK cell features in acute-treated individuals, and highlight the importance of examining effector cells not only in blood but also the lymphoid tissue compartment, where the reservoir mostly persists, and where these cells take on distinct phenotypic features.
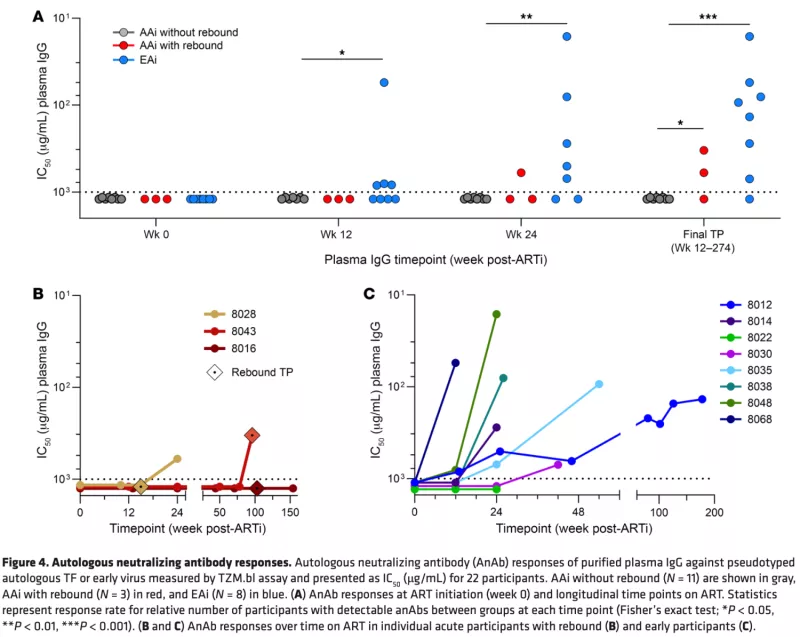
Autologous Neutralizing Antibody Responses After Antiretroviral Therapy in Acute and Early HIV-1
Early antiretroviral therapy initiation (ARTi) in HIV-1 restricts reservoir size and diversity while preserving immune function, potentially improving opportunities for immunotherapeutic cure strategies. For antibody-based cure approaches, the development of autologous neutralizing antibodies (anAbs) after acute/early ARTi is relevant but is poorly understood. We characterized antibody responses in our UCSF Treat Acute HIV cohort of 23 participants following ARTi in acute HIV (<60 days after acquisition) and early HIV (60–128 days after acquisition). Plasma virus sequences at the time of ARTi revealed evidence of escape from anAbs after early, but not acute, ARTi. HIV-1 envelopes representing the transmitted/founder virus(es) (acute ARTi) or escape variants (early ARTi) were tested for sensitivity to longitudinal plasma IgG. After acute ARTi, no anAb responses developed over months to years of suppressive ART. In 2 of the 3 acute ARTi participants who experienced viremia after ARTi, however, anAbs arose shortly thereafter. After early ARTi, anAbs targeting those early variants developed between 12 and 42 weeks of ART and continued to increase in breadth and potency thereafter. These findings indicate a threshold of virus replication (~60 days) required to induce anAbs, after which they continue to expand on suppressive ART to better target the range of reservoir variants.