Patient-focused in vivo studies enable the use of laboratory-based translational assays on rare clinical biospecimens to uncover potential causal relationships between pathogens, treatments, and disease outcomes.
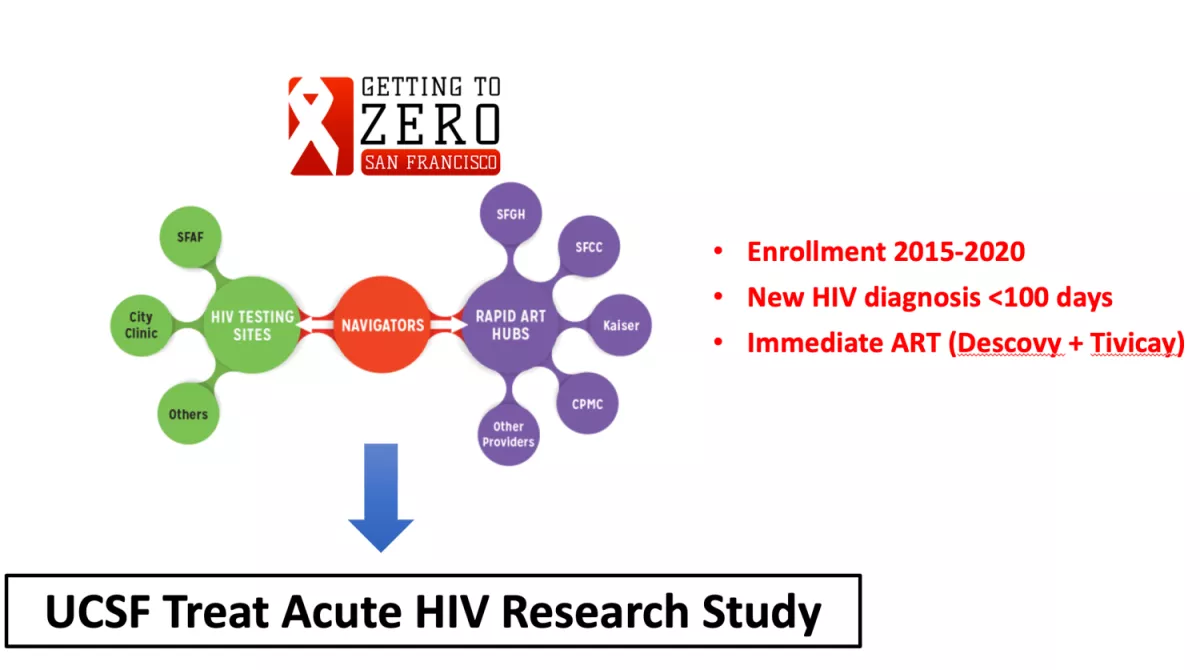
UCSF Treat Acute HIV Study
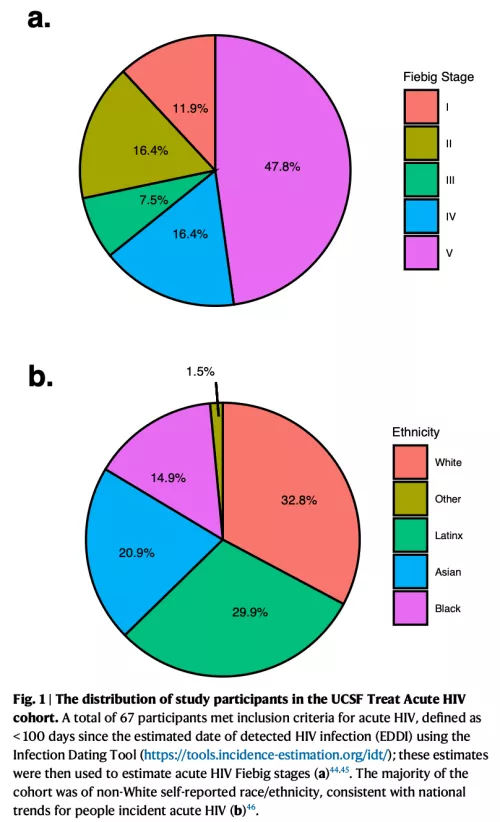
Given the potential individual and public health benefits of immediate antiretroviral therapy (ART) initiation, people with HIV (PWH) are now being treated as early as possible. Since the size of the HIV “reservoir” (cells in which HIV persist despite effective ART) is smaller with very early viral suppression and since the immune system is still relatively intact during this early stage, there is great interest in studying PWH during acute infection. In collaboration with our HIV/AIDS Ward 86 Clinic at the San Francisco General Hospital and the city of San Francisco’s campaign to identify and immediately treat newly diagnosed people with acute HIV, we enrolled PWH diagnosed within 100 days of estimated date of infection (https://clinicaltrials.gov/ct2/show/NCT02656511). Biospecimens collected as part of this study are now being leveraged to perform translational experiments that may provide direct insights into the pathophysiology of the earliest stages of acute HIV and identify virologic/host characteristics of a functional cure.
Effect of Methamphetamine on Residual Latent HIV Disease (EMRLHD) Study
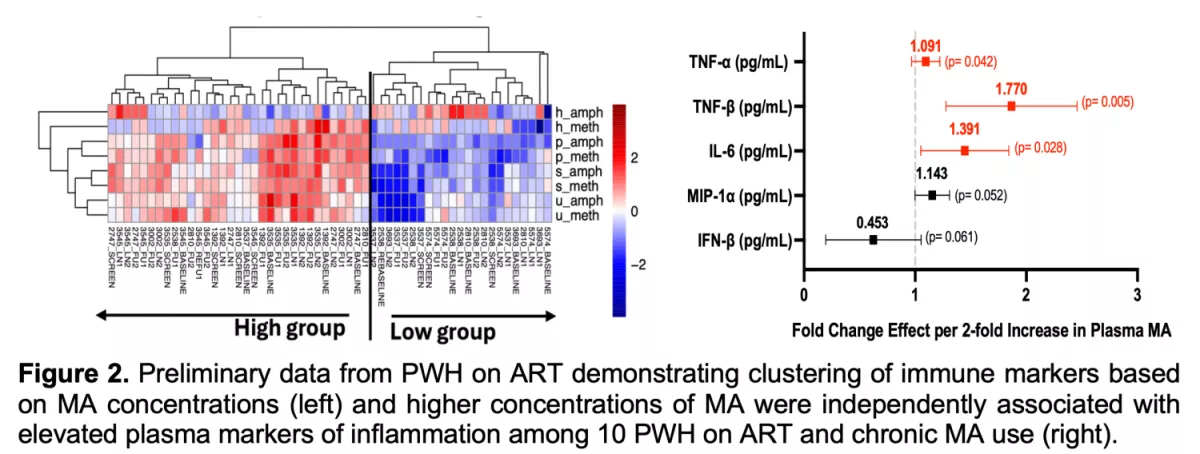
Globally, methamphetamine (MA) is the second most commonly used illegal substance, and in San Francisco, up to 39% of PWH report recent MA use. Despite higher burden of MA in PWH, very little is known about its impact on HIV persistence, viral-host responses, immune cell activation, and end-organ damage, particularly in the central nervous system (CNS) and cardiovascular system (CVS). No study to date has linked MA use with increased HIV transcription, tissue inflammation, immune dysregulation, and clinical outcomes during adequate ART suppression – which are critical understudied areas given an overall aging population of people living with HIV. Participants were enrolled from the EMRLHD cohort and included individuals reporting ongoing MA dependence disorder. Participants underwent longitudinal inguinal LN FNAs, blood collection, and MA concentration quantitation (plasma, serum, hair). We observed that PWH MA+ on ART have increased proinflammatory responses and enhanced HIV transcription, suggesting that long-term MA exposure is associated with increased innate immune and proinflammatory response, and viral transcription, even during adequate ART. We are now performing similar analyses from our EMRLHD clinical trial administering oral MA at FDA-approved doses (25 mg/day) to determine the short-term effect of MA on HIV transcription and host immune responses (https://clinicaltrials.gov/ct2/show/NCT03825536). Results from these studies may identify novel targets for reversing HIV latency, reducing inflammation, and personalizing future therapeutic strategies specific to the population of PWH who use MA.
Population Pharmacokinetics and Pharmacodynamic Modeling of FDA-Approved Drug to Induce Latent HIV Transcription Study
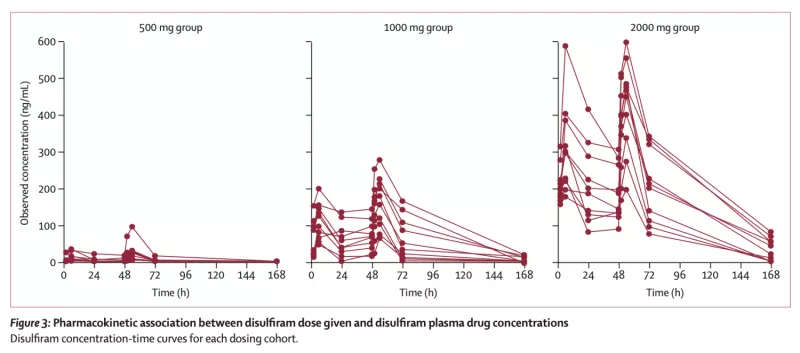
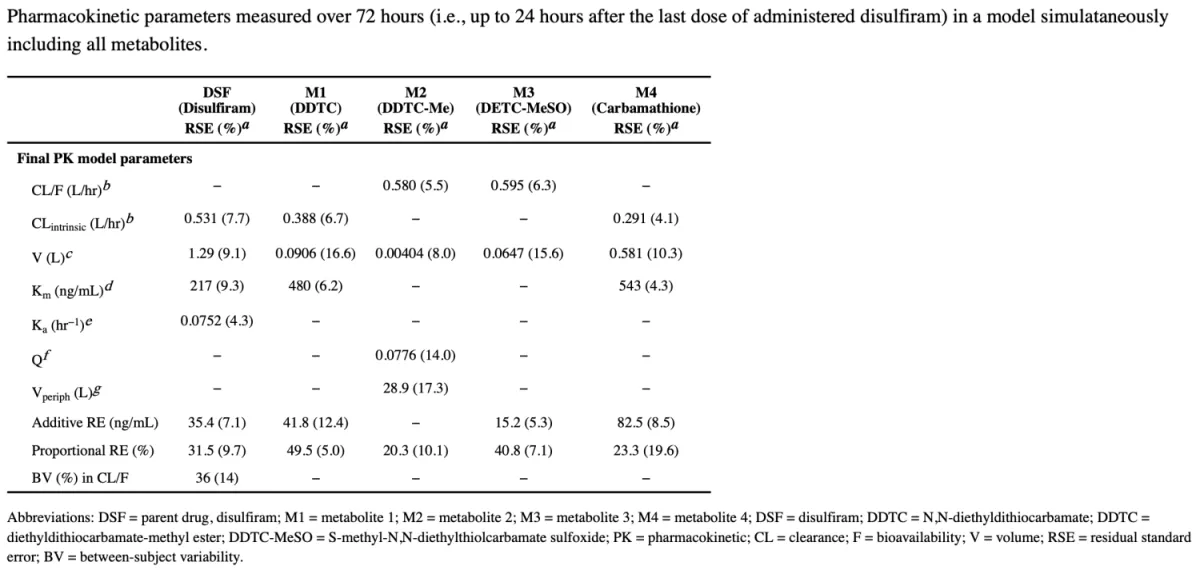
In a prospective phase 2 dose-escalation study, we tested disulfiram (an FDA-approved drug previously shown to activate HIV transcription in a primary T cell model of HIV latency) in PWH on ART. Participants were sequentially assigned to one of three dosing groups (500 mg, 1000 mg, and 2000 mg) and received disulfiram (DSF) daily for 3 days. The primary endpoint was safety and tolerability as well as change in cell-associated unspliced (CA-US) HIV RNA in CD4+ T cells as well as plasma HIV RNA by single copy assay. DSF was well tolerated and activated both HIV CA-US RNA and plasma RNA. We then performed population pharmacokinetc/pharmacodynamic (PK/PD) modeling using a seven-compartment model which demonstrated non-linear elimination kinetics. The fitted median area under the curve values (AUC0–72) were 3816, 8386, and 22331 mg*hr/L, respectively. Higher DSF exposure predicted greater increases in CA-US RNA (Emax=78%, AUC50=1600 mcg*hr/L, P=0.013) but not plasma HIV RNA. These results offer preliminary evidence supporting the exploration of existing FDA-approved drugs as potential components of future HIV cure strategies.
DISulfiram for COVID-19 (DISCO) Study
A hallmark of severe COVID-19 disease is immune system dysregulation called "cytokine storm." Multiple studies have reported that patients with severe disease demonstrate elevated levels of pro-inflammatory cytokines early in disease, such as interleukin (IL)-6. Recent data suggests multiple mechanisms by which disulfiram (DSF), an FDA-approved drug for the treatment of alcohol dependence disorder with a good safety profile and easy dosing schedule, may act on COVID-19 both as a direct antiviral as well as an indirect anti-inflammatory agent (e.g., via inhibition of the NLRP3 inflammasome and cytokine production by inhibiting gasdermin D pore formation). We have previously shown that disulfiram, given at high doses (2000 mg/day), is well-tolerated in PWH. Disulfiram has a short half-life ~7.5 hours with >90% of drug eliminated within 3 days post-dose, allowing quick reversal of any potential adverse effects. For the DISCO study (https://clinicaltrials.gov/ct2/show/NCT04485130), recent COVID-19+ individuals were randomized (2:1) to receive either oral disulfiram (1000 mg/day or 2000 mg/day) for 5 consecutive days or placebo to assess the effects of the drug on COVID-19 symptom severity, SARS-CoV-2 viral shedding, and inflammation. While the study ended early due to the widespread availability of FDA-approved nirmatrelvir/ritonavir (Paxlovid), analyses investigating the impact of DSF on host inflammation and immune activation as well as on SARS-CoV-2 viral loads from nasal swabs are planned.
Immunologic Response and Safety of Euphorbia Kansui: A Phase I Clinical Trial
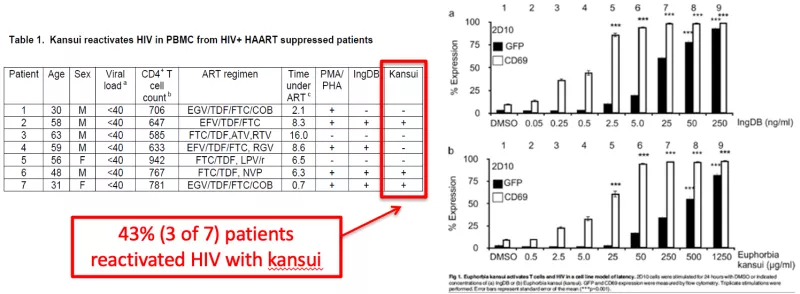
This unique proof-of-concept clinical trial tested the hypothesis that kansui, an herbal supplement commonly used in traditional Chinese medicine, is safe and well tolerated. The experiments of this study extend in vitro data demonstrating that kansui extract can reactivate virus from latently infected Jurkat cells to a clinical study evaluating the tolerability and biologic effect of kansui in PWH on ART. In collaboration with Dr. Adam Spivak at the University of Utah, kansui extract prepared as tea was given to study participants and assessed for safety and tolerability in a phase I randomized, placebo-controlled crossover trial (https://clinicaltrials.gov/ct2/show/NCT02531295). While the study was ended early due to the COVID-19 pandemic, analyses linking kansui dose with immunophenotyping and HIV reservoir data are in progress.